Resumen
En este trabajo se plantea una propuesta de laboratorio para valorar diferentes muestras de rocas ígneas y seleccionar, mediante análisis geoquímico y petrográfico, las mejores candidatas para exponerse a dióxido de carbono acuoso. Lo anterior sirve como guía a estudiantes de licenciatura y posgrado interesados en el tema de almacenamiento de CO2 que deseen conocer algunas de las técnicas de análisis geoquímico que se emplean en el laboratorio. Los protocolos experimentales diseñados fueron validados en el laboratorio por alumnos de los últimos semestres de la carrera de Ingeniería Petrolera y del Posgrado de la Facultad de Ingeniería de la UNAM, los cuales permitieron identificar los parámetros críticos para la mineralización de CO2 en rocas ígneas y olivino, que es un mineral común en las rocas ígneas máficas y se ha reportado como uno de los mejores prospectos para la mineralización de CO2.
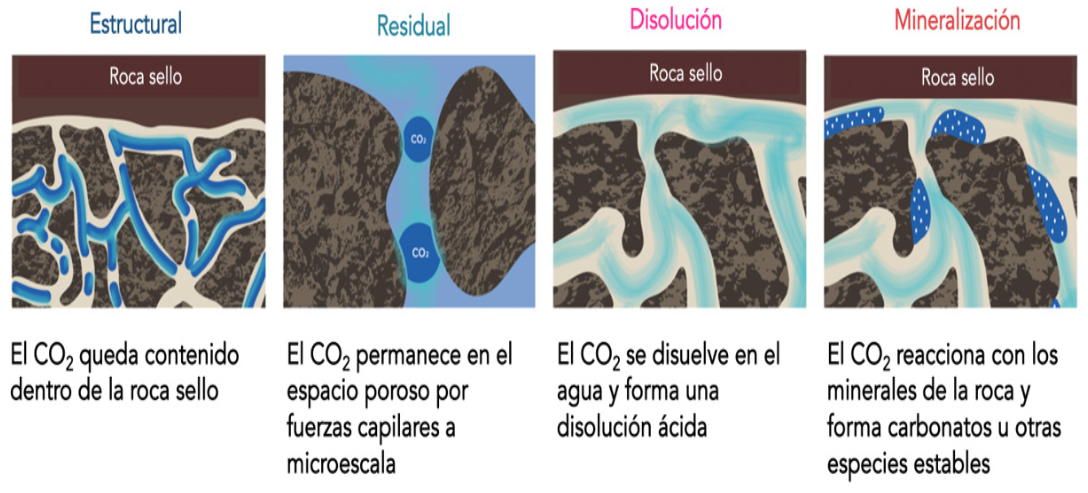
Las muestras de roca fueron expuestas a dióxido de carbono disuelto en agua, a una presión entre 5860 y 6550 kPa y una temperatura de 20 a 25 °C. Para confirmar la mineralización del dióxido de carbono se emplearon las técnicas de espectroscopía infrarroja, difracción de rayos X de polvos, microscopía electrónica de barrido y plasma de acoplamiento inductivo acoplado con espectrofotometría de emisión óptica. Se observó que, después de exponer el olivino a CO2 durante 21 y 60 días, se formaron bicarbonatos y carbonatos, respectivamente. Para el caso de las rocas ígneas, se identificaron bicarbonatos después de 30 días de exposición y se observó la liberación de iones Ca2+, Mg2+ y Fe3+ en el agua, que son críticos para la formación de carbonatos.
Aunque la mineralización de CO2 en este tipo de rocas se ha reportado previamente, es necesario continuar la investigación en este tema para elaborar una base de datos con las muestras más idóneas que favorezcan la mineralización de dióxido de carbono y se genere conocimiento de la capacidad de almacenamiento de CO2. Estas experiencias son relevantes para las y los estudiantes de licenciatura y posgrado afines a Ciencias de la Tierra, así como para complementar su formación en temas relacionados con el calentamiento global, la mitigación de emisiones de CO2 y la transición energética.
Citas
Akono, A.T., Druhan, J. L.; Davila, G.; Tsotsis, T.; Jessen, K.; Werth, C. J., (2019). A review of geochemical-mechanical impacts in geological carbon storage reservoirs. Greenhouse Gases-Science and Technology, 9 (3), 474-504. DOI: 10.1002/ghg.1870
Almazán-Mendoza, J. (2022). Identificación de parámetros físicos para la mineralización de CO2 en olivino y rocas ígneas. [Tesis de licenciatura, UNAM].
Cantú-Apodaca, E. (2018). Análisis de la factibilidad para la eliminación de dióxido de carbono mediante la formación de carbonatos a través de basaltos. [Tesis de licenciatura, UNAM].
Carbfix. (2023). How it works. Carbfix.Com. Enerdata. (2023). Producción energética total. Datos.enerdata. https://datos.enerdata.net/energia-total/produccion-energetica-mundial.html
Gadikota, G., Matter, J., Kelemen, P., Brady, P. v., Park, A. H. A. (2020). Elucidating the differences in the carbon mineralization behaviors of calcium and magnesium bearing alumino-silicates and magnesium silicates for CO2 storage. Fuel, 277, 0016-2361. https://doi.org/10.1016/j.fuel.2020.117900
Global CCS Institute (2020). Remove: Carbon Capture and Storage. globalccsinstitue.com.
Gómez-Tuena, A., Mori, L. y Straub, S. M. (2018). Geochemical and petrological insights into de tectonic origin of the Transmexican Volcanic Belt. Earth-Science Reviews, 183, 153–181.
IEA. (2022). CO2 Capture and Utilisation. iea.org.
Johnson, J. (2012). Thermal infrared spectra of experimentally shocked andesine anorthosite. Icarus, 221, 359-364. https://doi.org/10.1016/j.icarus.2012.08.012
Kwon S., Fan M., DaCosta H. F. M., y Russell A. G. (2011). Factors affecting the direct mineralization of CO2 with olivine. Journal of Environmental Sciences, 23(8), 1233–1239. https://doi.org/10.1016/S1001-0742(10)60555-4
Macías-Navarro L. (2020) Revista México Actúa. Número 1, 4 – 6.
McGrail, B. P., Schaef, H. T., Spane, F. A., Horner, J. A., Owen, A. T., Cliff, J. B., Qafoku, O., Thompson, C. J., y Sullivan, E. C. (2017). Wallula Basalt Pilot Demonstration Project: Post-injection Results and Conclusions. Energy Procedia, 114, 5783–5790. https://doi.org/10.1016/j.egypro.2017.03.1716
MeCCS. (s.f.). CCS y sostenibilidad. Meccs.Org.Mx. Consultado el 10 de febrero de 2023.
Naciones Unidas. (2023). COP26: Juntos por el planeta: un.org
Raza, A., Glatz, G., Gholami, R., Mahmoud, M., y Alafnan, S. (2022). Carbon mineralization and geological storage of CO2 in basalt: Mechanisms and technical challenges. Earth-Science Reviews, 229, 0012-8252. https://doi.org/10.1016/j.earscirev.2022.104036
Ritchie H., Roser M., y Rosado P. (2022) - "Energy". Ourwolrdindata.
Secretaría de Energía. (2018). Mapa de Ruta Tecnológica, CCUS.
Shukla, R., Rankith, P., Haque, A., Choi, X,. (2010). A review of studies on CO2 sequestration and caprock integrity. Fuel, 89, 2651-2664. https://doi.org/10.1016/j.fuel.2010.05.012
Snæbjörnsdóttir, S., Sigfússon, B., Marieni, C., Goldberg, D., Gislason, S. R., y Oelkers, E. H. (2020). Carbon dioxide storage through mineral carbonation. Nature Reviews Earth and Environment, 1(2), 90–102. https://doi.org/10.1038/s43017-019-0011-8
Staib, C., Zhang, T., Burrows, J., Gillespie, A., Havercroft, I., Rassool, D., Consoli, C., Liu, H., Erikson, J., Loria, P., Nambo, H., Wu, Y., Judge, C., y Gebremedhin, R. (2021). Global Status of CCS 2021.
White, S. K., Spane, F. A., Schaef, H. T., Miller, Q. R. S., White, M. D., Horner, J. A., y McGrail, B. P. (2020). Quantification of CO2 Mineralization at the Wallula Basalt Pilot Project. Environmental Science and Technology, 54 (22), 14609–14616. https://doi.org/10.1021/acs.est.0c05142
Young-Shin J., Giammar D., y C. J. Werth. (2012). Impacts of Geochemical Reactions on Geologic Carbon Sequestration. Environmental Science y Technology 47 (1), 3-8. https://doi.org/10.1021/es3027133
Zanettin B. (1984). Proposed New Chemical Classification of Volcanic Rocks. Journal of International Geoscience, 7 (4), 19-19. https://doi.org/10.18814/epiiugs/1984/v7i4/003
Zhenxue D., L. Xu, T. Xiao, B. McPherson, X. Zhang, L. Zheng, S. Dong, Z. Yang, M. R. Soltanian, C. Yang, W. Ampomah, W. S. Yin, T. Xu, D. Bacon, H. Viswanathan. (2020). Reactive chemical transport simulations of geologic carbon sequestration: Methods and applications. Earth-Science Reviews, 208, 0012-8252. https://doi.org/10.1016/j.earscirev.2020.103265.

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial 4.0.
Derechos de autor 2023 Universidad Nacional Autónoma de México